Portal:Minerals
The Minerals Portal

In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.
The geological definition of mineral normally excludes compounds that occur only in living organisms. However, some minerals are often biogenic (such as calcite) or organic compounds in the sense of chemistry (such as mellite). Moreover, living organisms often synthesize inorganic minerals (such as hydroxylapatite) that also occur in rocks.
The concept of mineral is distinct from rock, which is any bulk solid geologic material that is relatively homogeneous at a large enough scale. A rock may consist of one type of mineral or may be an aggregate of two or more different types of minerals, spacially segregated into distinct phases.
Some natural solid substances without a definite crystalline structure, such as opal or obsidian, are more properly called mineraloids. If a chemical compound occurs naturally with different crystal structures, each structure is considered a different mineral species. Thus, for example, quartz and stishovite are two different minerals consisting of the same compound, silicon dioxide. (Full article...)
Mineralogy is a subject of geology specializing in the scientific study of the chemistry, crystal structure, and physical (including optical) properties of minerals and mineralized artifacts. Specific studies within mineralogy include the processes of mineral origin and formation, classification of minerals, their geographical distribution, as well as their utilization. (Full article...)
Selected articles
-
Image 1Galena with minor pyrite
Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver.
Galena is one of the most abundant and widely distributed sulfide minerals. It crystallizes in the cubic crystal system often showing octahedral forms. It is often associated with the minerals sphalerite, calcite and fluorite. (Full article...) -
Image 2A lustrous crystal of zircon perched on a tan matrix of calcite from the Gilgit District of Pakistan
Zircon (/ˈzɜːrkɒn, -kən/) is a mineral belonging to the group of nesosilicates and is a source of the metal zirconium. Its chemical name is zirconium(IV) silicate, and its corresponding chemical formula is ZrSiO4. An empirical formula showing some of the range of substitution in zircon is (Zr1–y, REEy)(SiO4)1–x(OH)4x–y. Zircon precipitates from silicate melts and has relatively high concentrations of high field strength incompatible elements. For example, hafnium is almost always present in quantities ranging from 1 to 4%. The crystal structure of zircon is tetragonal crystal system. The natural color of zircon varies between colorless, yellow-golden, red, brown, blue, and green.
The name derives from the Persian zargun, meaning "gold-hued". This word is changed into "jargoon", a term applied to light-colored zircons. The English word "zircon" is derived from Zirkon, which is the German adaptation of this word. Yellow, orange, and red zircon is also known as "hyacinth", from the flower hyacinthus, whose name is of Ancient Greek origin. (Full article...) -
Image 3
Cinnabar (/ˈsɪnəˌbɑːr/; from Ancient Greek κιννάβαρι (kinnábari)), or cinnabarite (/ˌsɪnəˈbɑːraɪt/), also known as mercurblende is the bright scarlet to brick-red form of mercury(II) sulfide (HgS). It is the most common source ore for refining elemental mercury and is the historic source for the brilliant red or scarlet pigment termed vermilion and associated red mercury pigments.
Cinnabar generally occurs as a vein-filling mineral associated with volcanic activity and alkaline hot springs. The mineral resembles quartz in symmetry and it exhibits birefringence. Cinnabar has a mean refractive index near 3.2, a hardness between 2.0 and 2.5, and a specific gravity of approximately 8.1. The color and properties derive from a structure that is a hexagonal crystalline lattice belonging to the trigonal crystal system, crystals that sometimes exhibit twinning.
Cinnabar has been used for its color since antiquity in the Near East, including as a rouge-type cosmetic, in the New World since the Olmec culture, and in China since as early as the Yangshao culture, where it was used in coloring stoneware. In Roman times, cinnabar was highly valued as paint for walls, especially interiors, since it darkened when used outdoors due to exposure to sunlight.
Associated modern precautions for the use and handling of cinnabar arise from the toxicity of the mercury component, which was recognized as early as ancient Rome. (Full article...) -
Image 4
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond as a form of carbon is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of electricity, and insoluble in water. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. They are also the reason that diamond anvil cells can subject materials to pressures found deep in the Earth.
Because the arrangement of atoms in diamond is extremely rigid, few types of impurity can contaminate it (two exceptions are boron and nitrogen). Small numbers of defects or impurities (about one per million of lattice atoms) can color a diamond blue (boron), yellow (nitrogen), brown (defects), green (radiation exposure), purple, pink, orange, or red. Diamond also has a very high refractive index and a relatively high optical dispersion.
Most natural diamonds have ages between 1 billion and 3.5 billion years. Most were formed at depths between 150 and 250 kilometres (93 and 155 mi) in the Earth's mantle, although a few have come from as deep as 800 kilometres (500 mi). Under high pressure and temperature, carbon-containing fluids dissolved various minerals and replaced them with diamonds. Much more recently (hundreds to tens of million years ago), they were carried to the surface in volcanic eruptions and deposited in igneous rocks known as kimberlites and lamproites.
Synthetic diamonds can be grown from high-purity carbon under high pressures and temperatures or from hydrocarbon gases by chemical vapor deposition (CVD). Natural and synthetic diamonds are most commonly distinguished using optical techniques or thermal conductivity measurements.[citation needed] (Full article...) -
Image 5

Green fluorite with prominent cleavage
Cleavage, in mineralogy and materials science, is the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the crystal, which create smooth repeating surfaces that are visible both in the microscope and to the naked eye. If bonds in certain directions are weaker than others, the crystal will tend to split along the weakly bonded planes. These flat breaks are termed "cleavage". The classic example of cleavage is mica, which cleaves in a single direction along the basal pinacoid, making the layers seem like pages in a book. In fact, mineralogists often refer to "books of mica".
Diamond and graphite provide examples of cleavage. Each is composed solely of a single element, carbon. In diamond, each carbon atom is bonded to four others in a tetrahedral pattern with short covalent bonds. The planes of weakness (cleavage planes) in a diamond are in four directions, following the faces of the octahedron. In graphite, carbon atoms are contained in layers in a hexagonal pattern where the covalent bonds are shorter (and thus even stronger) than those of diamond. However, each layer is connected to the other with a longer and much weaker van der Waals bond. This gives graphite a single direction of cleavage, parallel to the basal pinacoid. So weak is this bond that it is broken with little force, giving graphite a slippery feel as layers shear apart. As a result, graphite makes an excellent dry lubricant.
While all single crystals will show some tendency to split along atomic planes in their crystal structure, if the differences between one direction or another are not large enough, the mineral will not display cleavage. Corundum, for example, displays no cleavage. (Full article...) -
Image 6

Borax (also referred to as sodium borate, tincal (/ˈtɪŋkəl/) and tincar (/ˈtɪŋkər/)) is a salt (ionic compound), a hydrated or anhydrous borate of sodium, with the chemical formula Na2H20B4O17.
It is a colorless crystalline solid that dissolves in water to make a basic solution.
It is commonly available in powder or granular form and has many industrial and household uses, including as a pesticide, as a metal soldering flux, as a component of glass, enamel, and pottery glazes, for tanning of skins and hides, for artificial aging of wood, as a preservative against wood fungus, and as a pharmaceutic alkalizer. In chemical laboratories, it is used as a buffering agent.
The terms tincal and tincar refer to native borax, historically mined from dry lake beds in various parts of Asia. (Full article...) -
Image 7

Tourmaline (/ˈtʊərməlɪn, -ˌliːn/ TOOR-mə-lin, -leen) is a crystalline silicate mineral group in which boron is compounded with elements such as aluminium, iron, magnesium, sodium, lithium, or potassium. This gemstone comes in a wide variety of colors.
The name is derived from the Sinhalese tōramalli (ටෝරමල්ලි), which refers to the carnelian gemstones. (Full article...) -
Image 8
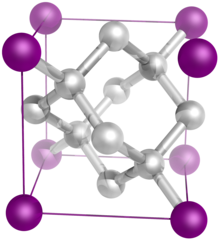
The diamond crystal structure belongs to the face-centered cubic lattice, with a repeated two-atom pattern.
In crystallography, a crystal system is a set of point groups (a group of geometric symmetries with at least one fixed point). A lattice system is a set of Bravais lattices. Space groups are classified into crystal systems according to their point groups, and into lattice systems according to their Bravais lattices. Crystal systems that have space groups assigned to a common lattice system are combined into a crystal family.
The seven crystal systems are triclinic, monoclinic, orthorhombic, tetragonal, trigonal, hexagonal, and cubic. Informally, two crystals are in the same crystal system if they have similar symmetries (though there are many exceptions). (Full article...) -
Image 9

Zeolite exhibited in the Estonian Museum of Natural History
Zeolite is a family of several microporous, crystalline aluminosilicate materials commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula Mn+
1/n(AlO
2)−
(SiO
2)
x・yH
2O where Mn+
1/n is either a metal ion or H+.
The term was originally coined in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that rapidly heating a material, believed to have been stilbite, produced large amounts of steam from water that had been adsorbed by the material. Based on this, he called the material zeolite, from the Greek ζέω (zéō), meaning "to boil" and λίθος (líthos), meaning "stone".
Zeolites occur naturally, but are also produced industrially on a large scale. As of December 2018[update], 253 unique zeolite frameworks have been identified, and over 40 naturally occurring zeolite frameworks are known. Every new zeolite structure that is obtained is examined by the International Zeolite Association Structure Commission (IZA-SC) and receives a three-letter designation. (Full article...) -
Image 10
The mineral pyrite (/ˈpaɪraɪt/ PY-ryte), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula FeS2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic luster and pale brass-yellow hue give it a superficial resemblance to gold, hence the well-known nickname of fool's gold. The color has also led to the nicknames brass, brazzle, and brazil, primarily used to refer to pyrite found in coal.
The name pyrite is derived from the Greek πυρίτης λίθος (pyritēs lithos), 'stone or mineral which strikes fire', in turn from πῦρ (pŷr), 'fire'. In ancient Roman times, this name was applied to several types of stone that would create sparks when struck against steel; Pliny the Elder described one of them as being brassy, almost certainly a reference to what is now called pyrite.
By Georgius Agricola's time, c. 1550, the term had become a generic term for all of the sulfide minerals. (Full article...) -
Image 11

Rutile is an oxide mineral composed of titanium dioxide (TiO2), the most common natural form of TiO2. Rarer polymorphs of TiO2 are known, including anatase, akaogiite, and brookite.
Rutile has one of the highest refractive indices at visible wavelengths of any known crystal and also exhibits a particularly large birefringence and high dispersion. Owing to these properties, it is useful for the manufacture of certain optical elements, especially polarization optics, for longer visible and infrared wavelengths up to about 4.5 micrometres. Natural rutile may contain up to 10% iron and significant amounts of niobium and tantalum.
Rutile derives its name from the Latin rutilus ('red'), in reference to the deep red color observed in some specimens when viewed by transmitted light. Rutile was first described in 1803 by Abraham Gottlob Werner using specimens obtained in Horcajuelo de la Sierra, Madrid (Spain), which is consequently the type locality. (Full article...) -
Image 12
Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of OH−, F− and Cl− ion, respectively, in the crystal. The formula of the admixture of the three most common endmembers is written as Ca10(PO4)6(OH,F,Cl)2, and the crystal unit cell formulae of the individual minerals are written as Ca10(PO4)6(OH)2, Ca10(PO4)6F2 and Ca10(PO4)6Cl2.
The mineral was named apatite by the German geologist Abraham Gottlob Werner in 1786, although the specific mineral he had described was reclassified as fluorapatite in 1860 by the German mineralogist Karl Friedrich August Rammelsberg. Apatite is often mistaken for other minerals. This tendency is reflected in the mineral's name, which is derived from the Greek word ἀπατάω (apatáō), which means to deceive. (Full article...) -
Image 13
Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe2+Fe3+2O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. With the exception of extremely rare native iron deposits, it is the most magnetic of all the naturally occurring minerals on Earth. Naturally magnetized pieces of magnetite, called lodestone, will attract small pieces of iron, which is how ancient peoples first discovered the property of magnetism.
Magnetite is black or brownish-black with a metallic luster, has a Mohs hardness of 5–6 and leaves a black streak. Small grains of magnetite are very common in igneous and metamorphic rocks.
The chemical IUPAC name is iron(II,III) oxide and the common chemical name is ferrous-ferric oxide. (Full article...) -
Image 14Malachite from the Democratic Republic of the Congo
Malachite is a copper carbonate hydroxide mineral, with the formula Cu2CO3(OH)2. This opaque, green-banded mineral crystallizes in the monoclinic crystal system, and most often forms botryoidal, fibrous, or stalagmitic masses, in fractures and deep, underground spaces, where the water table and hydrothermal fluids provide the means for chemical precipitation. Individual crystals are rare, but occur as slender to acicular prisms. Pseudomorphs after more tabular or blocky azurite crystals also occur. (Full article...) -
Image 15Deep green isolated fluorite crystal resembling a truncated octahedron, set upon a micaceous matrix, from Erongo Mountain, Erongo Region, Namibia (overall size: 50 mm × 27 mm, crystal size: 19 mm wide, 30 g)
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon.
The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite.
Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite has anomalous partial dispersion, that is, its refractive index varies with the wavelength of light in a manner that differs from that of commonly used glasses, so fluorite is useful in making apochromatic lenses, and particularly valuable in photographic optics. Fluorite optics are also usable in the far-ultraviolet and mid-infrared ranges, where conventional glasses are too opaque for use. Fluorite also has low dispersion, and a high refractive index for its density. (Full article...) -
Image 16
Graphite (/ˈɡræfaɪt/) is a crystalline allotrope (form) of the element carbon. It consists of many stacked layers of graphene, typically in the excess of hundreds of layers. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on a large scale (1.3 million metric tons per year in 2022) for uses in many critical industries including refractories (50%), lithium-ion batteries (18%), foundries (10%), lubricants (5%), among others (17%). Under extremely high pressures and extremely high temperatures it converts to diamond. Graphite's low cost, thermal and chemical inertness and characteristic conductivity of heat and electricity finds numerous applications in high energy and high temperature processes. (Full article...) -
Image 17

Kaolinite (/ˈkeɪ.ələˌnaɪt, -lɪ-/ KAY-ə-lə-nyte, -lih-; also called kaolin) is a clay mineral, with the chemical composition Al2Si2O5(OH)4. It is a layered silicate mineral, with one tetrahedral sheet of silica (SiO4) linked through oxygen atoms to one octahedral sheet of alumina (AlO6).
Kaolinite is a soft, earthy, usually white, mineral (dioctahedral phyllosilicate clay), produced by the chemical weathering of aluminium silicate minerals like feldspar. It has a low shrink–swell capacity and a low cation-exchange capacity (1–15 meq/100 g).
Rocks that are rich in kaolinite, and halloysite, are known as kaolin (/ˈkeɪ.əlɪn/) or china clay. In many parts of the world kaolin is colored pink-orange-red by iron oxide, giving it a distinct rust hue. Lower concentrations of iron oxide yield the white, yellow, or light orange colors of kaolin. Alternating lighter and darker layers are sometimes found, as at Providence Canyon State Park in Georgia, United States.
Kaolin is an important raw material in many industries and applications. Commercial grades of kaolin are supplied and transported as powder, lumps, semi-dried noodle or slurry. Global production of kaolin in 2021 was estimated to be 45 million tonnes, with a total market value of US $4.24 billion. (Full article...) -
Image 18A ruby crystal from Dodoma Region, Tanzania
Ruby is a pinkish red to blood-red colored gemstone, a variety of the mineral corundum (aluminium oxide). Ruby is one of the most popular traditional jewelry gems and is very durable. Other varieties of gem-quality corundum are called sapphires. Ruby is one of the traditional cardinal gems, alongside amethyst, sapphire, emerald, and diamond. The word ruby comes from ruber, Latin for red. The color of a ruby is due to the element chromium.
Some gemstones that are popularly or historically called rubies, such as the Black Prince's Ruby in the British Imperial State Crown, are actually spinels. These were once known as "Balas rubies".
The quality of a ruby is determined by its color, cut, and clarity, which, along with carat weight, affect its value. The brightest and most valuable shade of red, called blood-red or pigeon blood, commands a large premium over other rubies of similar quality. After color follows clarity: similar to diamonds, a clear stone will command a premium, but a ruby without any needle-like rutile inclusions may indicate that the stone has been treated. Ruby is the traditional birthstone for July and is usually red/pinker than garnet, although some rhodolite garnets have a similar pinkish hue to most rubies. The world's most valuable ruby to be sold at auction is the Sunrise Ruby, which sold for US$34.8 million. (Full article...) -
Image 19Beachy Head is a part of the extensive Southern England Chalk Formation.
Chalk is a soft, white, porous, sedimentary carbonate rock. It is a form of limestone composed of the mineral calcite and originally formed deep under the sea by the compression of microscopic plankton that had settled to the sea floor. Chalk is common throughout Western Europe, where deposits underlie parts of France, and steep cliffs are often seen where they meet the sea in places such as the Dover cliffs on the Kent coast of the English Channel.
Chalk is mined for use in industry, such as for quicklime, bricks and builder's putty, and in agriculture, for raising pH in soils with high acidity. It is also used for "blackboard chalk" for writing and drawing on various types of surfaces, although these can also be manufactured from other carbonate-based minerals, or gypsum. (Full article...) -
Image 20A sample of andesite (dark groundmass) with amygdaloidal vesicles filled with zeolite. Diameter of view is 8 cm.
Andesite (/ˈændəzaɪt/) is a volcanic rock of intermediate composition. In a general sense, it is the intermediate type between silica-poor basalt and silica-rich rhyolite. It is fine-grained (aphanitic) to porphyritic in texture, and is composed predominantly of sodium-rich plagioclase plus pyroxene or hornblende.
Andesite is the extrusive equivalent of plutonic diorite. Characteristic of subduction zones, andesite represents the dominant rock type in island arcs. The average composition of the continental crust is andesitic. Along with basalts, andesites are a component of the Martian crust.
The name andesite is derived from the Andes mountain range, where this rock type is found in abundance. It was first applied by Christian Leopold von Buch in 1826. (Full article...) -
Image 21
Opal is a hydrated amorphous form of silica (SiO2·nH2O); its water content may range from 3% to 21% by weight, but is usually between 6% and 10%. Due to the amorphous (chemical) physical structure, it is classified as a mineraloid, unlike crystalline forms of silica, which are considered minerals. It is deposited at a relatively low temperature and may occur in the fissures of almost any kind of rock, being most commonly found with limonite, sandstone, rhyolite, marl, and basalt.
The name opal is believed to be derived from the Sanskrit word upala (उपल), which means 'jewel', and later the Greek derivative opállios (ὀπάλλιος).
There are two broad classes of opal: precious and common. Precious opal displays play-of-color (iridescence); common opal does not. Play-of-color is defined as "a pseudo chromatic optical effect resulting in flashes of colored light from certain minerals, as they are turned in white light." The internal structure of precious opal causes it to diffract light, resulting in play-of-color. Depending on the conditions in which it formed, opal may be transparent, translucent, or opaque, and the background color may be white, black, or nearly any color of the visual spectrum. Black opal is considered the rarest, while white, gray, and green opals are the most common. (Full article...) -
Image 22The 423-carat (85 g) blue Logan Sapphire
Sapphire is a precious gemstone, a variety of the mineral corundum, consisting of aluminium oxide (α-Al2O3) with trace amounts of elements such as iron, titanium, cobalt, lead, chromium, vanadium, magnesium, boron, and silicon. The name sapphire is derived from the Latin word sapphirus, itself from the Greek word sappheiros (σάπφειρος), which referred to lapis lazuli. It is typically blue, but natural "fancy" sapphires also occur in yellow, purple, orange, and green colors; "parti sapphires" show two or more colors. Red corundum stones also occur, but are called rubies rather than sapphires. Pink-colored corundum may be classified either as ruby or sapphire depending on the locale. Commonly, natural sapphires are cut and polished into gemstones and worn in jewelry. They also may be created synthetically in laboratories for industrial or decorative purposes in large crystal boules. Because of the remarkable hardness of sapphires – 9 on the Mohs scale (the third-hardest mineral, after diamond at 10 and moissanite at 9.5) – sapphires are also used in some non-ornamental applications, such as infrared optical components, high-durability windows, wristwatch crystals and movement bearings, and very thin electronic wafers, which are used as the insulating substrates of special-purpose solid-state electronics such as integrated circuits and GaN-based blue LEDs. Sapphire is the birthstone for September and the gem of the 45th anniversary. A sapphire jubilee occurs after 65 years. (Full article...) -
Image 23

Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula CaSO4·2H2O. It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, drywall and blackboard or sidewalk chalk. Gypsum also crystallizes as translucent crystals of selenite. It forms as an evaporite mineral and as a hydration product of anhydrite. The Mohs scale of mineral hardness defines gypsum as hardness value 2 based on scratch hardness comparison.
Fine-grained white or lightly tinted forms of gypsum known as alabaster have been used for sculpture by many cultures including Ancient Egypt, Mesopotamia, Ancient Rome, the Byzantine Empire, and the Nottingham alabasters of Medieval England. (Full article...) -
Image 24

A crystalline solid: atomic resolution image of strontium titanate. Brighter spots are columns of strontium atoms and darker ones are titanium-oxygen columns.
Crystallography is the branch of science devoted to the study of molecular and crystalline structure and properties. The word crystallography is derived from the Ancient Greek word κρύσταλλος (krústallos; "clear ice, rock-crystal"), and γράφειν (gráphein; "to write"). In July 2012, the United Nations recognised the importance of the science of crystallography by proclaiming 2014 the International Year of Crystallography.
Crystallography is a broad topic, and many of its subareas, such as X-ray crystallography, are themselves important scientific topics. Crystallography ranges from the fundamentals of crystal structure to the mathematics of crystal geometry, including those that are not periodic or quasicrystals. At the atomic scale it can involve the use of X-ray diffraction to produce experimental data that the tools of X-ray crystallography can convert into detailed positions of atoms, and sometimes electron density. At larger scales it includes experimental tools such as orientational imaging to examine the relative orientations at the grain boundary in materials. Crystallography plays a key role in many areas of biology, chemistry, and physics, as well new developments in these fields. (Full article...) -
Image 25
Beryl (/ˈbɛrəl/ BERR-əl) is a mineral composed of beryllium aluminium silicate with the chemical formula Be3Al2Si6O18. Well-known varieties of beryl include emerald and aquamarine. Naturally occurring hexagonal crystals of beryl can be up to several meters in size, but terminated crystals are relatively rare. Pure beryl is colorless, but it is frequently tinted by impurities; possible colors are green, blue, yellow, pink, and red (the rarest). It is an ore source of beryllium. (Full article...)
Selected mineralogist
-
Image 1Frederick Eugene Wright (October 16, 1877 – August 25, 1953) was an American optical scientist and geophysicist. He was the second president of the Optical Society of America from 1918-1919. (Full article...)
-
Image 2

Ignacy Domeyko or Domejko, pseudonym: Żegota (Spanish: Ignacio Domeyko, Spanish pronunciation: [iɣˈnasjo ðoˈmejko]; 31 July 1802 – 23 January 1889) was a Polish geologist, mineralogist, educator, and founder of the University of Santiago, in Chile. Domeyko spent most of his life, and died, in his adopted country, Chile.
After a youth passed in partitioned Poland, Domeyko participated in the Polish–Russian War 1830–31. Upon Russian victory, he was exiled, spending part of his life in France (where he had gone with a fellow Philomath, Polish poet Adam Mickiewicz) before eventually settling in Chile, whose citizen he became. (Full article...) -
Image 3
Johann Nepomuk von Fuchs (15 May 1774 – 5 March 1856) was a German chemist and mineralogist, and royal Bavarian privy councillor. (Full article...) -
Image 4Oskar Alexander Richard Büttner (28 September 1858 – 1927) was a German botanist and mineralogist who was involved in the exploration of the Congo Basin. (Full article...)
-
Image 5
Emil Wilhelm Cohen (12 October 1842 – 13 April 1905) was a German mineralogist and petrographer, born in Jutland. (Full article...) -
Image 6

Bust of Frederic Cailliaud
Frédéric Cailliaud (9 June 1787 – 1 May 1869) was a French naturalist, mineralogist and conchologist. He was born, and died, in Nantes, where he was the curator of the Natural History Museum of Nantes from 1836 to 1869.
He travelled in Egypt, Nubia, and Ethiopia, collecting minerals and making observations. He was a part of the military expedition that his patron Viceroy Muhammad Ali sent south to conquer the Kingdom of Sennar, but also marched further into Fazogli where Caillaud searched for outcroppings of gold while the commander Ismail, son of Muhammad Ali, enslaved locals and slaughtered all who resisted him. Although he failed to find any sizeable deposits of gold in the mountains along the modern Sudan-Ethiopia border, he did make a sufficiently detailed survey of the area to be published after he returned to France in 1827. (Full article...) -
Image 7Vesselina Vassileva Breskovska (Bulgarian: Веселина Василева Бресковска) (December 6, 1928, Granit, Stara Zagora Province, Bulgaria – August 12, 1997, Sofia, Bulgaria) was a 20th-century Bulgarian geologist, mineralogist and crystallographer. (Full article...)
-
Image 8
Auguste Bravais (French pronunciation: [oɡyst bʁavɛ]; 23 August 1811, Annonay, Ardèche – 30 March 1863, Le Chesnay, France) was a French physicist known for his work in crystallography, the conception of Bravais lattices, and the formulation of Bravais law. Bravais also studied magnetism, the northern lights, meteorology, geobotany, phyllotaxis, astronomy, statistics and hydrography.
He studied at the Collège Stanislas in Paris before joining the École Polytechnique in 1829, where he was a classmate of groundbreaking mathematician Évariste Galois, whom Bravais actually beat in a scholastic mathematics competition. Towards the end of his studies he became a naval officer, and sailed on the Finistere in 1832 as well as the Loiret afterwards. He took part in hydrographic work along the Algerian Coast. He participated in the Recherche expedition and helped the Lilloise in Spitzbergen and Lapland. (Full article...) -
Image 9Wilhelm Hermann Julius Eitel (6 May 1891, Frankfurt am Main – 20 July 1979, United States) was a German-American scientist. (Full article...)
-
Image 10Frank Thomas Matthews White (7 February 1909 – 5 July 1971) was an Australian mining and metallurgical engineer and mineral science educator. His career included appointments in Australia, Fiji, Malaysia, and Canada.
In 1968, he initiated an Institute for Mineral Industry Research. Two buildings, including a geodesic dome, were constructed to house the institute at the mineral-rich Gault Estate in Mont-Saint-Hilaire, Québec, where ventilation research and simulations were conducted. The initiative laid the groundwork for establishing an Institute of Occupational Health and Safety at McGill in 1974. (Full article...) -
Image 11Ian Stuart Edward Carmichael FRS (29 March 1930 –26 August 2011) was a British-born American igneous petrologist and volcanologist who established extensive quantitative methods for research in the thermodynamics of magma. (Full article...)
-
Image 12

Félix Pisani (1831–1920)
Félix Pisani (28 April 1831, Constantinople – 7 November 1920, Paris) was a French chemist and mineralogist.
He was born in Istanbul, where his Venetian father worked in the Russian diplomatic service. Beginning in 1854, he studied chemistry in Paris at a private school run by Charles Frédéric Gerhardt (1816–1856). (Full article...) -
Image 13

Franz Ernst Brückmann (27 September 1697 – 21 March 1753) was a German mineralogist born at Marienthal near Helmstedt. Having qualified as a physician in 1721, he practised at Braunschweig and afterwards at Wolfenbüttel (from 1728). In 1747 he was appointed medical assessor in Braunschweig.
His leisure time was given up to natural history, and especially to mineralogy and botany. He appears to have been the first to introduce the term "oolithus" to rocks that resemble in structure the roe of a fish; whence the terms "oolite" and "oolitic". He died at Wolfenbüttel. (Full article...) -
Image 14
Charles Friedel (French: [ʃaʁl fʁidɛl]; 12 March 1832 – 20 April 1899) was a French chemist and mineralogist. (Full article...) -
Image 15Portrait by Joseph Karl Stieler (1843)
Friedrich Wilhelm Heinrich Alexander von Humboldt (14 September 1769 – 6 May 1859) was a German polymath, geographer, naturalist, explorer, and proponent of Romantic philosophy and science. He was the younger brother of the Prussian minister, philosopher, and linguist Wilhelm von Humboldt (1767–1835). Humboldt's quantitative work on botanical geography laid the foundation for the field of biogeography, while his advocacy of long-term systematic geophysical measurement pioneered modern geomagnetic and meteorological monitoring. Humboldt and Carl Ritter are both regarded as the founders of modern geography as they established it as an independent scientific discipline.
Between 1799 and 1804, Humboldt travelled extensively in the Americas, exploring and describing them for the first time from a non-Spanish European scientific point of view. His description of the journey was written up and published in several volumes over 21 years. (Full article...) -
Image 16David Forbes FRS (6 September 1828 – 5 December 1876) was a Manx mineralogist, metallurgist, and chemist. (Full article...)
-
Image 17

Quintino Sella (Italian pronunciation: [kwinˈtino ˈsɛlla]; 7 July 1827 – 14 March 1884) was an Italian politician, economist and mountaineer. (Full article...) -
Image 18

Ernst Erhard Schmid
Ernst Erhard Friedrich Wilhelm Schmid (22 May 1815 in Hildburghausen – 16 February 1885 in Jena) was a German paleontologist. He was the son of law professor Karl Ernst Schmid (1774–1852).
He studied natural sciences at the universities of Jena and Vienna, receiving his doctorate in 1839. In 1843 he became an associate professor at Jena, where with Matthias Jakob Schleiden, he founded a physiological institute. At the institute he dealt with subjects that included mineralogy, geology, chemistry and physics. In 1856 he was appointed a professor of natural sciences at the University of Jena. (Full article...) -
Image 19

Adolph Knopf (December 2, 1882 – November 23, 1966) was an American geologist. Educated at the University of California, Berkeley, he held professional appointments at the United States Geological Survey, Yale University, and Stanford University. He was primarily a petrologist and mineralogist, though later in his career contributed to geochronology. He performed much of his field work in the western United States, investigating mineral deposits in Alaska, the Boulder Batholith in Montana, and the Gold Country of California.
Knopf was a member of the National Academy of Sciences and the American Academy of Arts and Sciences. He served as president of the Geological Society of America in 1944 and received its Penrose Medal in 1959. His second wife, Eleanora Knopf, was a notable geologist and frequent collaborator. (Full article...) -
Image 20

Liebisch's Portrait
Theodor Liebisch (29 April 1852, Breslau – 9 February 1922, Berlin) was a German mineralogist and crystallographer. (Full article...) -
Image 21Adam August Krantz (6 December 1808 in Neumarkt in Schlesien – 6 April 1872 in Berlin) was a German mineralogist. (Full article...)
-
Image 22

Carl Linnaeus (23 May 1707 – 10 January 1778), also known after ennoblement in 1761 as Carl von Linné, was a Swedish biologist and physician who formalised binomial nomenclature, the modern system of naming organisms. He is known as the "father of modern taxonomy". Many of his writings were in Latin; his name is rendered in Latin as Carolus Linnæus and, after his 1761 ennoblement, as Carolus a Linné.
Linnaeus was the son of a curate and was born in Råshult, in the countryside of Småland, southern Sweden. He received most of his higher education at Uppsala University and began giving lectures in botany there in 1730. He lived abroad between 1735 and 1738, where he studied and also published the first edition of his Systema Naturae in the Netherlands. He then returned to Sweden where he became professor of medicine and botany at Uppsala. In the 1740s, he was sent on several journeys through Sweden to find and classify plants and animals. In the 1750s and 1760s, he continued to collect and classify animals, plants, and minerals, while publishing several volumes. By the time of his death in 1778, he was one of the most acclaimed scientists in Europe. (Full article...) -
Image 23
Berend George Escher (4 April 1885 – 11 October 1967) was a Dutch geologist.
Escher had a broad interest, but his research was mainly on crystallography, mineralogy and volcanology. He was a pioneer in experimental geology. He was a half-brother of the artist M. C. Escher, and had some influence on his work due to his knowledge of crystallography. M.C. Escher created a woodcut ex libris for his brother 'Beer' with a stylized image of a volcano around 1922 (Bool number 91). (Full article...) -
Image 24
James De Carle Sowerby (5 June 1787 – 26 August 1871) was a British mineralogist, botanist, and illustrator. He received an education in chemistry.
Sowerby was born in London, the son of botanical artist James Sowerby (1757–1822), and his wife, Anne de Carle (1764–1815). He continued his father's work and published, together with his brother George Brettingham Sowerby I, the latter volumes of the Mineral Conchology of Great Britain, begun by their father. (Full article...) -
Image 25
Hartvig Caspar Christie in 1873
Hartvig Caspar Christie (1 December 1826 – 3 March 1873) was a Norwegian mineralogist and physicist. (Full article...)
Related portals
Get involved
For editor resources and to collaborate with other editors on improving Wikipedia's Minerals-related articles, see WikiProject Rocks and minerals.
General images
-
Image 2Mohs Scale versus Absolute Hardness (from Mineral)
-
Image 4Schist is a metamorphic rock characterized by an abundance of platy minerals. In this example, the rock has prominent sillimanite porphyroblasts as large as 3 cm (1.2 in). (from Mineral)
-
Image 5Sphalerite crystal partially encased in calcite from the Devonian Milwaukee Formation of Wisconsin (from Mineral)
-
Image 7Epidote often has a distinctive pistachio-green colour. (from Mineral)
-
Image 8Perfect basal cleavage as seen in biotite (black), and good cleavage seen in the matrix (pink orthoclase). (from Mineral)
-
Image 9Muscovite, a mineral species in the mica group, within the phyllosilicate subclass (from Mineral)
-
Image 11Hübnerite, the manganese-rich end-member of the wolframite series, with minor quartz in the background (from Mineral)
-
Image 12Native gold. Rare specimen of stout crystals growing off of a central stalk, size 3.7 x 1.1 x 0.4 cm, from Venezuela. (from Mineral)
-
Image 14Asbestiform tremolite, part of the amphibole group in the inosilicate subclass (from Mineral)
-
Image 15Red cinnabar (HgS), a mercury ore, on dolomite. (from Mineral)
-
Image 16Gypsum desert rose (from Mineral)
-
Image 21Pink cubic halite (NaCl; halide class) crystals on a nahcolite matrix (NaHCO3; a carbonate, and mineral form of sodium bicarbonate, used as baking soda). (from Mineral)
-
Image 22Mohs hardness kit, containing one specimen of each mineral on the ten-point hardness scale (from Mohs scale)
-
Image 23Diamond is the hardest natural material, and has a Mohs hardness of 10. (from Mineral)
-
Image 24When minerals react, the products will sometimes assume the shape of the reagent; the product mineral is termed a pseudomorph of (or after) the reagent. Illustrated here is a pseudomorph of kaolinite after orthoclase. Here, the pseudomorph preserved the Carlsbad twinning common in orthoclase. (from Mineral)
-
Image 25An example of elbaite, a species of tourmaline, with distinctive colour banding. (from Mineral)
-
Image 26Black andradite, an end-member of the orthosilicate garnet group. (from Mineral)
Did you know ...?
- ... that while bultfonteinite (pictured) was discovered as early as 1903, the mineral wasn't described until 1932?
- ... that the minerals armalcolite, pyroxferroite and tranquillityite were discovered in lunar rocks?
- ... that taaffeite, one of the world's rarest gemstones, is the first mineral known to contain both beryllium and magnesium as essential components?
- ... that the newly discovered mineral krotite likely was one of the earliest minerals formed in the Solar System?
Subcategories
Topics
| Overview | ||
|---|---|---|
| Common minerals | ||
Ore minerals, mineral mixtures and ore deposits | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ores |
| ||||||||
| Deposit types | |||||||||
| Borates | |||||
|---|---|---|---|---|---|
| Carbonates | |||||
| Oxides |
| ||||
| Phosphates | |||||
| Silicates | |||||
| Sulfides | |||||
| Other |
| ||||
| Micas |
|
|---|---|
| Talcs |
|
| Pyrophyllite series |
|
| Kaolinites |
|
| Serpentines |
|
| Corrensites | |
| Smectites and vermiculite family |
|
| Chlorites | |
| Allophanes |
|
| Sepiolites |
|
| Pyrosmalites |
|
| Stilpnomelanes |
|
Structural groups mainly; based on rruff.info/ima, modified | |
| Crystalline | |||||||
|---|---|---|---|---|---|---|---|
| Cryptocrystalline | |||||||
| Amorphous | |||||||
| Miscellaneous | |||||||
| Notable varieties |
| ||||||
| Oxide minerals |
| ||||
|---|---|---|---|---|---|
| Silicate minerals | |||||
| Other | |||||
Gemmological classifications by E. Ya. Kievlenko (1980), updated | |||||||||
| Jewelry stones |
| ||||||||
| Jewelry-Industrial stones |
| ||||||||
| Industrial stones |
| ||||||||
Mineral identification | |
|---|---|
| "Special cases" ("native elements and organic minerals") |
|
|---|---|
| "Sulfides and oxides" |
|
| "Evaporites and similars" |
|
| "Mineral structures with tetrahedral units" (sulfate anion, phosphate anion, silicon, etc.) |
|
Associated Wikimedia
The following Wikimedia Foundation sister projects provide more on this subject:
-
Commons
Free media repository -
Wikibooks
Free textbooks and manuals -
Wikidata
Free knowledge base -
Wikinews
Free-content news -
Wikiquote
Collection of quotations -
Wikisource
Free-content library -
Wikiversity
Free learning tools -
Wiktionary
Dictionary and thesaurus