Melperonum
 |
Cave: notitiae huius paginae nec praescriptiones nec consilia medica sunt. |
| Cognitores | |
|---|---|
| ChemSpider | 14646 |
| PubChem | 15387 |
| DrugBank | DB09224 |
| Natura chemica | |
| |

| |
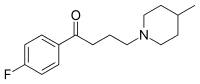
| Formula summarum | C 16H 22FNO |
| Massa molaris | 263.35 g/mol |
| Natura pharmacologica | |
| Codex ATC | N05AD03 (WHO) |
| Tempus semivitae biologicum | 3-4 horae |
| Metabolismus | iecore (hepaticus) |
| Excretio | renibus (70%) |
| Ad usum therapeuticum | |
| Applicatio | per os, i.m. |
Melperonum est substantia sedativum atque antipsychotica levior, ideoque praecipue ad therapiam insomniam praescriptum.
Natura Melperoni
Natura chemica
Melperonum ut benperidolum et haloperidolum et triperidolum est butyrophenonorum (butyrophenonum est 1-phenylbutan-1-onum). Structura chemica melperoni est 4-fluorum-4-(4-methyl-piperidino)-butyrophenonum.
Massa molaris est 263.35 g/mol.
Natura pharmacologica
Melperono effectus sedativus est. Codex ATC est N05AD03.
Pharmacodynamica
Melperonum potissime D3-, deinde alpha2-, alpha1-, D3-, 5-HT2A-, receptoria obsident.
| Receptorium | Affinitas ligandi Ki (nM)[1] |
Annotatio |
|---|---|---|
| serotonini 5-HT1A | 2,200 | |
| serotonini 5-HT1D | 3,400 | |
| serotonini 5-HT2A | 230 | Serotonini receptoriorum affinitas altissima |
| serotonini 5-HT2C | 2,100 | |
| serotonini 5-HT6 | 1,254 | |
| serotonini 5-HT7 | 578 | |
| adrenergici α1 | 180 | |
| adrenergici α2 | 180 | |
| acetylcholini M1 | >10,000 | exigue |
| acetylcholini M2 | 2,400 | |
| acetylcholini M3 | >10,000 | exigue |
| acetylcholini M4 | 4,400 | |
| acetylcholini M5 | >10,000 | exigue |
| dopamini D2 | 194 | Haloperidolum: 1.55 (fortius) |
| dopamini D3 | 8.95 | Dopamini receptoriorum affinitas altissima; Haloperidolum: 0.74 (fortius) |
| dopamini D4 | 555 | |
| histamini H1 | 580 | Haloperidolum: 1,800 (levius) |
Pharmacocinetica
Effectus primi transitus magnus. Tempus semivitae biologicum .[2] 3-4 horae est. Excretio est per urinas et biles.
Effectus Melperoni
Effectus non grati
Cum uso melperoni animum advertere ad effectus secundarios et interactiones necesse est.
Effectus secundarii
Exempli (!) sunt:
- Dystonia
- Akathisia
- Hypersalivatio
- Miosis
- Prolongatio intervalli QT
Interactiones
Melperonum est inhibitor CYP2D6.[3][4][5]
Nexus interni
Notae
- ↑ PDSP.
- ↑ Goldbook.
- ↑ Gahr, M; Gastl, R; Kölle, MA; Schönfeldt-Lecuona, C; Freudenmann, RW (2012). "Successful treatment of schizophrenia with melperone augmentation in a patient with phenotypic CYP2D6 ultrarapid metabolization: a case report". Journal of Medical Case Reports 6 (1): 49 (Anglice).
- ↑ Köhnke, MD; Lutz, U; Wiatr, G; Schwärzler, F; Weller, B; Schott, K; Buchkremer, G (April 2006). "Cytochrome P450 2D6 dependent metabolization of risperidone is inhibited by melperone". European Journal of Clinical Pharmacology 62 (4): 333–334 (Anglice).
- ↑ Grözinger, M; Dragicevic, A; Hiemke, C; Shams, M; Müller, MJ; Härtter, S (January 2003). "Melperone is an inhibitor of the CYP2D6 catalyzed O-demethylation of venlafaxine". Pharmacopsychiatry 36 (1): 3–6 (Anglice).
